Written by: Neelam Dharmadhikari
The case revolved around the U.S. Patent No. 7,604,929 (“’929 patent”) filed by In Vitro Inc. The patent also covers the product LiverPoolTM (Pooled multi-cryopreserved hepatocyte). The assignee are manufacturers specialized in vitro products for the study of metabolism, drug-drug interactions, and toxicity in drug discovery and development. The company was formerly known as In Vitro Technologies, Inc. and changed its name to Celsis In Vitro, Inc. in April 2007. As of July 1, 2013, Celsis In Vitro, Inc. was acquired by Bioreclamation, LLC.
The case filed in the District Court and won by CellzDirect Inc. under 35 U.S.C. § 101 that is invalidity of patent for subject matter eligibility. The appellee is a bioscience company, provides hepatocyte-based cell products and contracts laboratory services to bio/pharmaceutical companies. As of January 31, 2008, CellzDirect, Inc. operates as a subsidiary of Life Technologies Corporation. Thermo Fisher Scientific Corporation acquired the company in 2014 and used the Life Technologies brand name for a family of biotechnology products and services from Feb 2014 to 2015. From July 2015 Brand name was retired.
Let us see about the subject matter of the patent from which the case raised. It was a method of cryopreservation of hepatocytes. Hepatocytes are nothing but type of liver cells having tremendous applications in various studies including Drug metabolizing enzyme activity (stability, clearance, species comparison, metabolite profiling and ID), Time-dependent metabolism Drug-drug interaction, Plated drug transporter assays, Toxicity of drug candidates etc.
These hepatocytes have very short lifespan and thus require cryopreservation under temperature range of -90°C to -196°C. Before using they need to thaw at the temperature of 37°Cto 42°C. The controlled process of freezing and thawing and again freezing is required to store the cells in live form. But the post-thaw cell viability is greater than 50% and preferably 70% or more. Repeated Freeze-thaw facilitates Pooled Hepatocyte preparation.
Facts of the case:
In Vitro Inc. (IVT) sued CellzDirect, Inc. and Invitrogen Corporation (collectively, “LTC”) for infringing the ‘929 Patent. LTC filed a motion for summary judgment of invalidity under 35 U.S.C. § 101 and 112.
The Northern District Court of Illinois applied the Supreme Court’s two-step framework for determining Patent Eligibility under § 101 (Mayo V. Prometheus Labs, 2012). The District Court granted LTC’s motion, finding the ’929 patent invalid under § 101, dismissing the case with prejudice. The District Court ordered a preliminary injunction against CellzDirect Inc., Invitrogen Corporation, and Life Technologies (the Defendants) due to their infringement of U.S. Patent No. 7,604,929 (the Liverpool patent) in 2010. In response to this, IVT appealed to the Federal Circuit seeking for review of the district court’s summary judgment. Federal Court affirmed District Court’s order of Preliminary Injunction against CellzDirect, Inc. and Invitrogen Corporation, now Life Technologies Corporation (LTC) in the year 2012. Hearings Continued till July 2016 and on 5 July 2016 Court declared that the patent is not “directed to” a patent as an Ineligible building block of human ingenuity. Vacated and remanded Case for further proceedings and Costs to Appellant.
For reference here is the definition of patentable invention under US Code
- 35 U.S.C. § 101: Inventions patentable
Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefor, subject to the conditions and requirements of this title.
Mayo/Alice Test for Subject Matter Eligibility
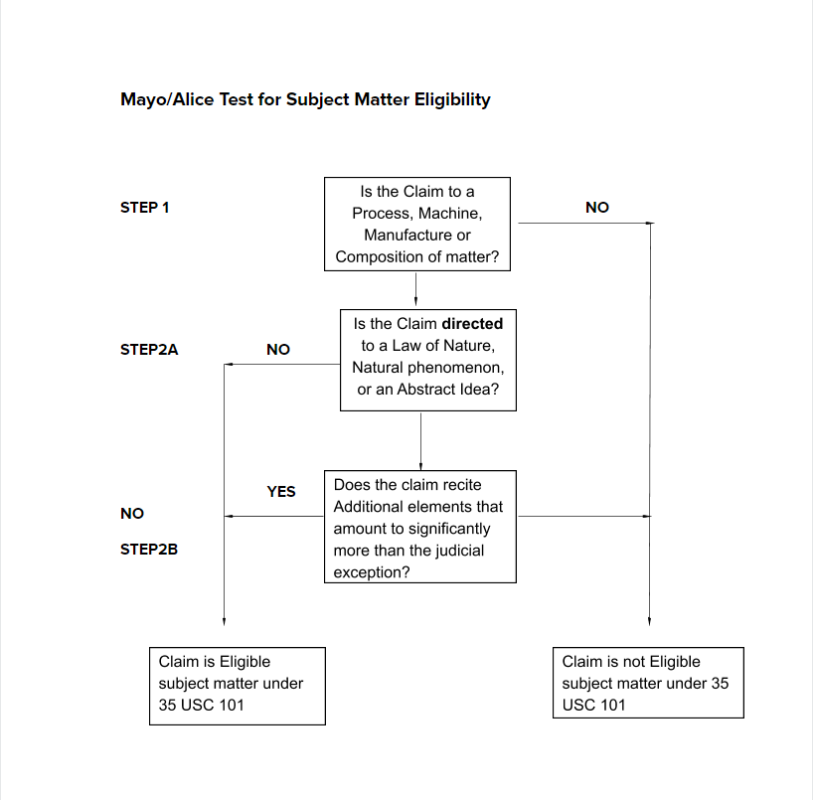
The US Patent & Trademark Office (USPTO) announced revised guidance on how it will review patents and applications for subject-matter eligibility with respect to Step 2A i.e. whether the claim is directed to one of the judicial exceptions to patent eligibility includes three sections and, in effect, separate the analysis required under Alice Step 2A into two inquiries (Prong One and Prong Two) The revised guidance went into effect on 7 January 2019 and applies to all patents and patent applications.
Comparison of Mayo, Myriad and In Vitro Claims
The below-given comparison between three cases wherein the Court gave its judgment based on Subject matter eligibility under 35 USC 101.
- Mayo US Patent No. 6,355,623
Claim 1: A method of optimizing therapeutic efficacy for treatment of an immune-mediated gastrointestinal disorder, comprising: (a) administering a drug providing 6-thioguanine to a subject having said immune-mediated gastrointestinal disorder, and (b) determining the level of 6-thioguanine in said subject having said immune-mediated gastrointestinal disorder,
wherein the level of 6-thioguanine less than about 230 pmol per 8×108 red blood cells indicates a need to increase the amount of said drug subsequently administered to said subject and
wherein the level of 6-thioguanine greater than about 400 pmol per 8×108 red blood cells indicates a need to decrease the amount of said drug subsequently administered to said subject.
Court’s Observation:
Justice Breyer explained that this claim sets forth a law of nature “namely, relationships between concentrations of certain metabolites in the blood and the likelihood that a dosage of a thiopurine drug will provide ineffective or cause harm.”
Whether the claims do significantly more than simply describe these natural relations. To put the matter more precisely, do the patent claims add enough to their statements of the correlations to allow the processes they describe to qualify as patent-eligible processes that apply natural laws?”
Myriad Genetics, US Patent No. 5,693,473 A
Claim 1: An isolated DNA comprising an altered BRCA1 DNA having at least one of the alterations set forth in Tables 12A, 14, 18, or 19 with the proviso that the alteration is not a deletion of four nucleotides corresponding to base numbers 4184-4187 in SEQ. ID. NO:1.
Court’s Observation:
There are no method claims before the Court. Had Myriad created an innovative method of manipulating genes while searching for the BRCA1 and BRCA2 genes, it could possibly have sought a method patent. But the processes used by Myriad to isolate DNA at the time of Myriad’s patents “were well understood, widely used, and fairly uniform”.
Court held the genes and the information they encode are not patent-eligible under §101 simply because they have been isolated from the surrounding genetic material.
In Vitro Inc. US Patent No. 7,604,929
Claim 1: A method of producing a desired preparation of multi-cryopreserved hepatocytes, said hepatocytes, being capable of being frozen and thawed at least two times, and in which greater than 70% of the hepatocytes of said preparation are viable after the final thaw, said method comprising: (A) subjecting hepatocytes that have been frozen and thawed to density gradient fractionation to separate viable hepatocytes from non-viable hepatocytes,
(B)recovering the separated viable hepatocytes, and
(C)cryopreserving the recovered viable hepatocytes to thereby form said desired preparation of hepatocytes without requiring a density gradient step after thawing the hepatocytes for the second time, wherein the hepatocytes are not plated between the first and second cryopreservation, and wherein greater than 70% of the hepatocytes of said preparation are viable after District Court’s Observation:
According to the District Court, the claim was “directed to an ineligible law of nature” (the “discovery” that hepatocytes could be subjected to multiple freeze/thaw cycles) under step 1. The District Court found the claim failed to recite an “inventive concept” under step 2 of the Mayo/Alice test because it simply “reapplied a well-understood freezing process”.
Federal Court disagreed with the lower Court’s finding of “hepatocytes are capable of surviving multiple freeze-thaw cycles.”
Findings of Federal Circuit:
- The claims are simply not directed to the ability of hepatocytes to survive multiple freeze-thaw cycles. Rather, are directed to a new and useful laboratory technique for preserving hepatocytes.
- This type of constructive process, carried out by an artisan to achieve “a new and useful end,” is precisely the type of claim that is eligible for patenting.
Claim 5: The method of claim 1, wherein said preparation comprises a pooled preparation of hepatocytes of multiple sources.
Court’s Opinion over Claim 5:
The individual steps of freezing and thawing were well known, but a process of preserving hepatocytes by repeating those steps was itself far from routine and conventional.
Claim 5 adds to the method, reciting “a pooled preparation of hepatocytes of multiple sources.” achieved a notable advance over prior art techniques for preserving hepatocytes.
Impact and Practical Applications of Rapid Litigation V. CellzDirect Case
- The case has helped Patent Applicants from Biotechnology and Software field by providing guidelines to clear Two-step tests.
- The decision helped to establish that, the two-step rule of subject matter eligibility guidance and training examples are consistent with the Federal Circuit’s decisions in the case.
- During drafting, emphasize the new and improved end result throughout ;
1) the specification to provide support for arguments that the claimed method is not directed to a patent-ineligible concept.
2) During prosecution and Litigation, focus arguments on the new and improved end result.
3) Address the second step of the Mayo analysis by focusing on the claimed method steps as a combination and how it differs as compared to prior art.
References:
- US 7,604,929B2 Daniel Dryden, James Hardy
US Update: New 101 Guidance, Kyu Yun Kim and iothy P. McAulty, CIPA Journal, April 2019